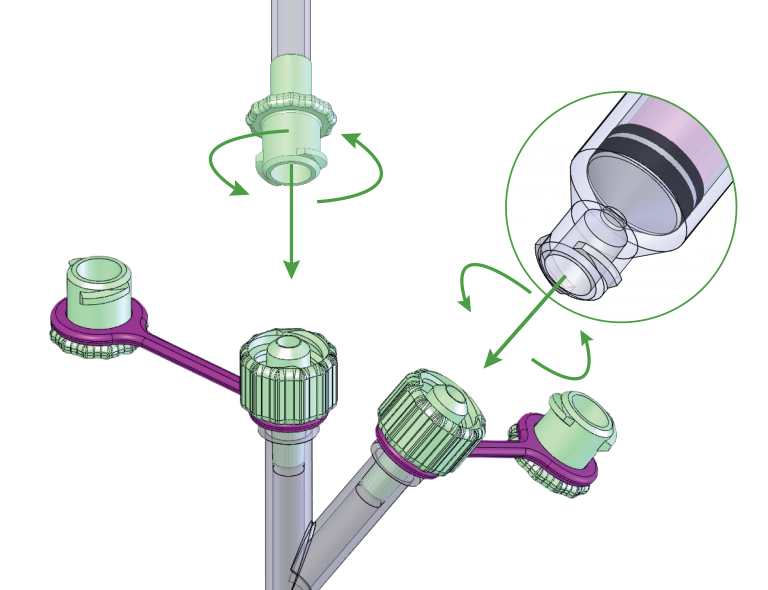
AMT is Prepared for ENFit®
ISO 80369-3, better known as ENFit®, is a global industry change that has been transitioning into the enteral nutrition market. We understand that this may be a difficult transition for many of our customers and we are with you every step of the way.
Products compatible with the new ISO 80369-3 standard (or ENFit®) will be available from AMT.
Visit StayConnected.org for additional information on the ENFit® System.
AMT would like to remind our customers that we will continue to offer a complete Legacy Line and a complete ENFit® Line, pursuant to FDA regulations.
As of January 1, 2022 all GEDSA Manufacturer Members (AMT is not a member) will no longer manufacture transitions sets and adaptors sold separately from other devices, as stated in the July 1, 2021 Revised ENFit® Connector Conversion Schedule.
AMT is not a member of GEDSA and, while we believe in their efforts and align with their messaging of patient safety, our position has not changed.
ENFit® is a registered trademark of GEDSA, Inc. or its affiliates.