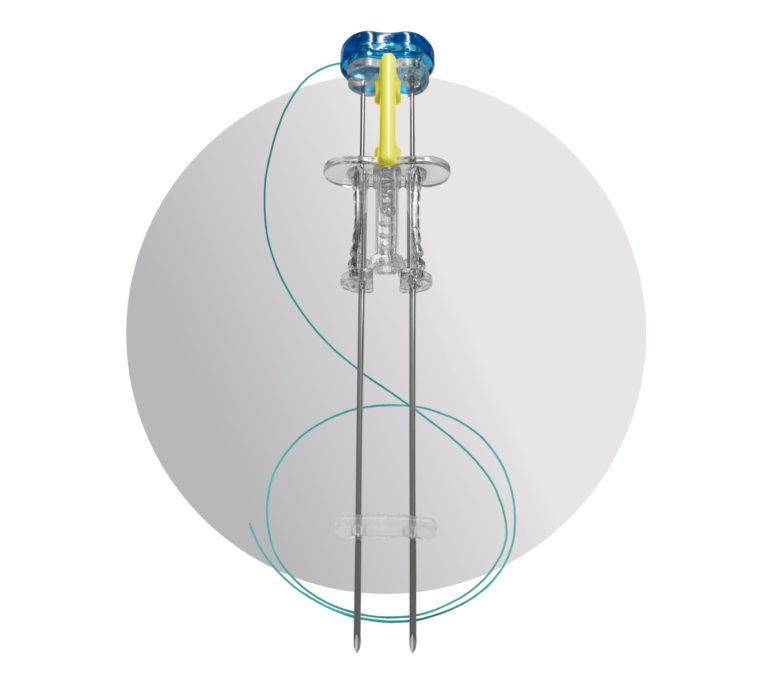
The Benefits of a U-Stitch with the Ease of a T-Fastener
Developed for surgeon ease of use, the ATLAS® U-Stitch uses exclusive magnetic technology to quickly and safely form a u-stitch within the hollow viscus. After proper placement, the organ is secured to the abdominal wall. Designed with patient comfort and safety in mind, this device was created to decrease the possibility of adverse events during -pexy procedures.
- Increased simplicity of placing a u-stitch.
- Strong securement without metal.
- Consistent and precise suture placement with fixed needle spacing.
- 36% smaller area, 20% lower profile, and > 77% softer bumper versus the leading competitor.1
ATLAS® U-Stitch brochure.
Hollow Viscus Attachment to the Abdominal Wall
What is the ATLAS® U-Stitch?
The ATLAS® U-Stitch builds upon decades of hollow viscus attachment techniques – and improves them for modern surgical practice. This innovative suture delivery system utilizes magnets to form a u-stitch within a hollow viscus to secure the organ to the abdominal wall. After proper placement, only the suture remains, which then can be tied over a soft, silicone bumper – completely metal-free. Following this procedure, an appropriate interventional catheter will be placed according to patient needs.
This device is designed to provide a more broadly applicable and faster alternative to existing manual u-stitches and t-fasteners.
In Saini et al. it was found that adhering the viscus to the abdominal wall ahead of the placement of an interventional catheter, compared to not performing the pexy, helped ensure tube patency and prevent possible adverse events such as peritonitis.2
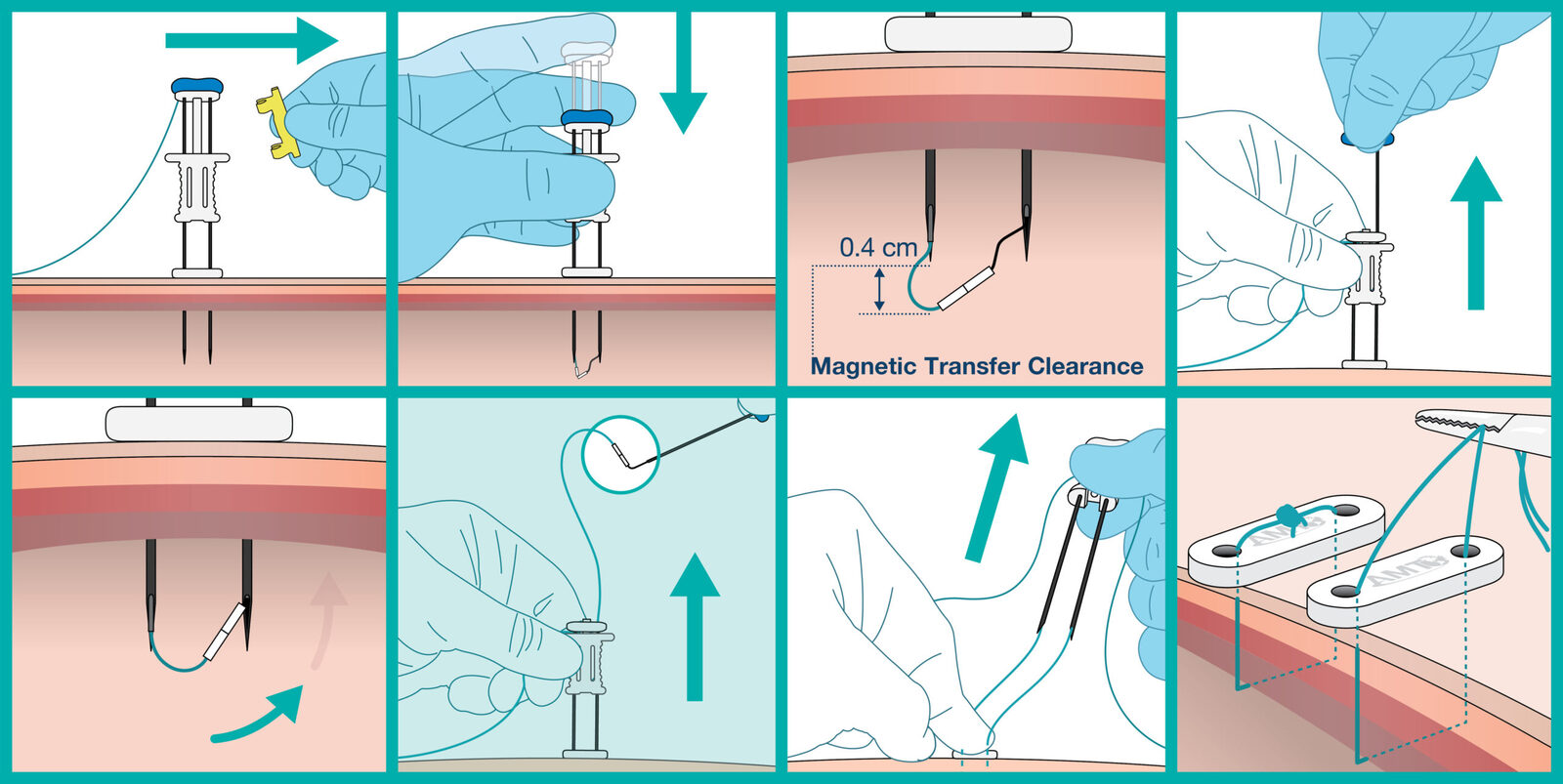
Prompt Placement of the ATLAS® U-Stitch*
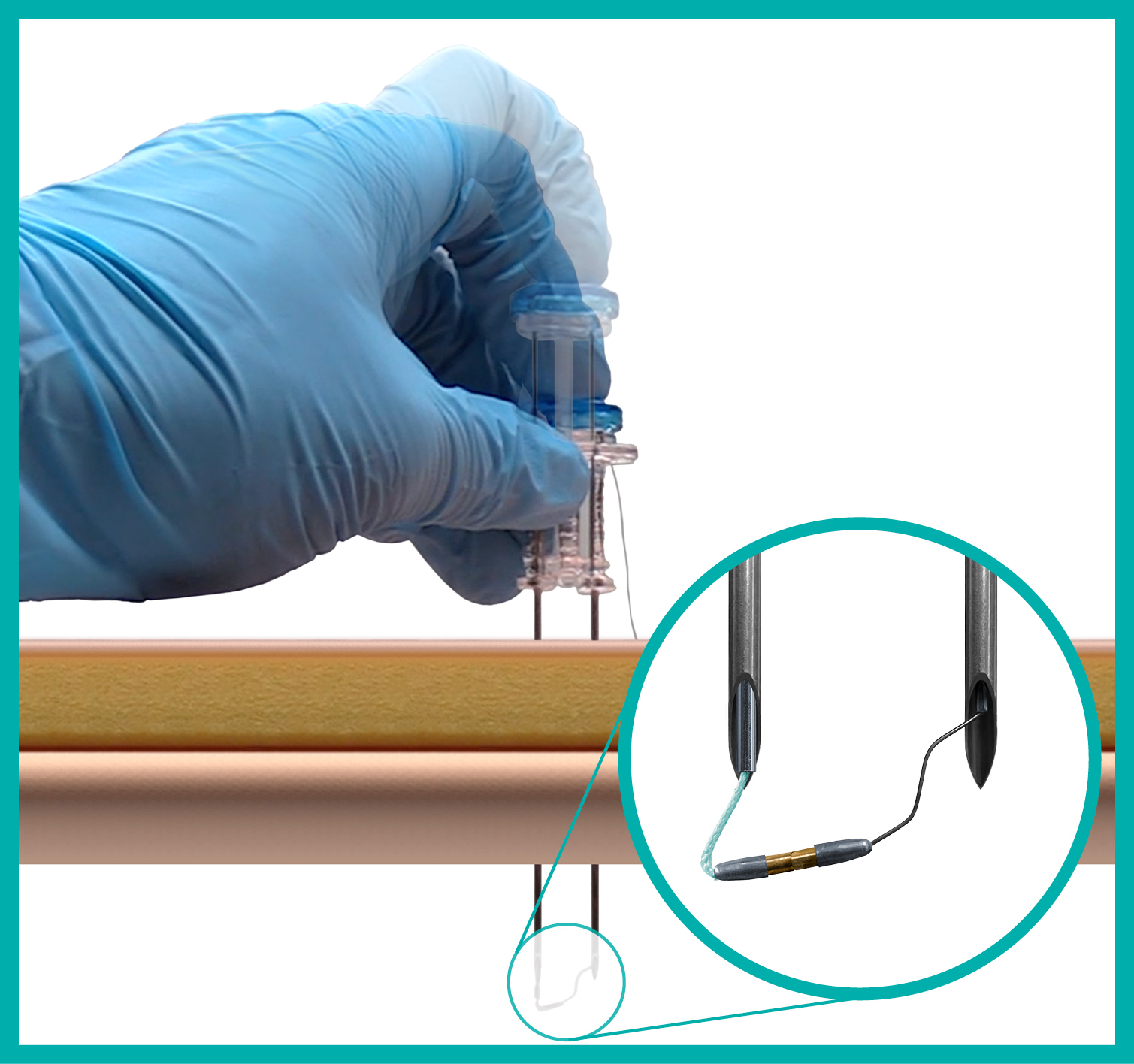
Suture Delivery System with Magnetic Technology
Instead of using a manual lasso or metal anchor, the ATLAS® U-Stitch utilizes rare-earth magnets to complete the suture loop within the hollow viscus. Once the suture loop is complete and the ATLAS® U-Stitch is removed, no metal is left behind, eliminating the need for it to pass through the body.
*This is NOT a substitute for the IFU/DFU (Instructions for Use/Directions for Use).
Benefits for Providers + Patients
Designed to Decrease the Possibility of Adverse Events.
As compared to manual u-stitches:
- The ATLAS® U-Stitch can be used in patients with an abdominal thickness up to 5 cm. Manual u-stitches have limited applicability to differing body types, especially those outside of early childhood.
- The design of the ATLAS® U-Stitch ensures that the suture bite is consistent across placements, whereas manual u-stitches are often inconsistent.
As compared to t-fasteners:
The ATLAS® U-Stitch has flexibility, allowing the clinician to choose whether to leave an external bumper. T-fasteners leave no options when deciding on external retention and require clinicians to cut above the skin to remove the bolster, which increases the risk of accidentally nicking the skin upon bumper removal.
Device Anatomy
Specially Designed Needles
The ATLAS® U-Stitch features two parallel introducer needles made of medical-grade stainless steel with cutting-style tips. These tips are designed to reduce the need to nick the skin before insertion of the device. Additionally, these needles feature an anti-coring heel designed to avoid coring of the fascia during insertion.
Exclusive Suture
The suture left after placement is a 3-0 braided permanent suture. This suture is FDA cleared to remain in place for up to 14 days following placement and does not lose strength over the placement period.
Comfortable Bumper
This soft and flexible silicone bumper can be used to separate the suture knot from the top of the skin. Its low-profile design is meant to maximize comfort for the patient.
Safety Aspects
The device has safety features that guard against accidental early deployments and associated risks. These include a needle cover to sheath the needle tips and a device lock to prevent depression of the advancement hub prematurely.
Device Application Examples:

The Stomach
Gastropexy

The Large + Small Intestines
Enteropexy + Cecopexy
The Bladder
Cystopexy
Gastrostomy Tube Initial Placement with the ATLAS® U-Stitch, AMT I.P. Kit, and the AMT MiniONE® – a winning combination!
With the introduction of the ATLAS® U-Stitch device – you can now complete a gastrostomy tube initial placement from start to finish with AMT products. This ensures that your team and your patient benefit from the full range of exclusive AMT benefits.

Part Numbers & Ordering Information
Healthcare professionals can order with established NET 30 terms or prepayment/credit card.
Fax or e-mail our Customer Service department to place your order!
Fax: 440-717-4200
Email: CS@AppliedMedical.net
Frequently Asked Questions
AMT has provided this information as an educational resource tool. This is not intended as a substitute for professional medical care.
Your FIRST source of information should be your healthcare provider.
Following proper placement, are the external bumper and suture MR Safe?
After removing the delivery device, the external bumper and suture are MR Safe.
How many ATLAS® U-Stitch Devices are ideal for proper viscus attachment?
The ideal number of devices needed is dependent on the procedure performed and physician preference.
Is the external bumper required for proper placement?
No, the external bumper may be placed or removed, solely pursuant to the physician’s preference.
What is the minimum internal clearance needed for the magnetic connection to occur?
0.4 cm.
What is the working length of the parallel introducer needles?
5 cm.
Can this device be used in child and adult populations?
Yes, this device is FDA cleared and indicated for use in both child and adult populations.
How long can the bumper remain in place?
The device is FDA cleared for up to 14 days of continuous use.
How is this device packaged?
This device is presented in a double sterile package with an exterior pouch and interior tray. With this in mind, this product can come one device per tray, or two devices per tray, and in box multiples of one or five. See the part number chart above for order information.
1. Based on internal testing of the ATLAS U-Stitch versus the leading competitor t-fastener device.↩
2. Saini S, Mueller PR, Gaa J, Briggs SE, Hahn PF, Forman BH, Tung GA, Silverman SG, Lee MJ, Morrison MC: Percutaneous gastrostomy with gastropexy: experience in 125 patients. AJR Am J Roentgenol 1990;154:1003–1006.↩
3. Sydnor, R. H., Schriber, S. M., & Kim, C. Y. (2014). T-fastener migration after percutaneous gastropexy for transgastric enteral tube insertion. Gut and liver, 8(5), 495–499. https://doi.org/10.5009/gnl13204↩
Questions? Contact us Today!
Full contact form
"*" indicates required fields
 Order Info
Order Info