A Superior Airway Management System!
Direct Oxygen Delivery, Simplified
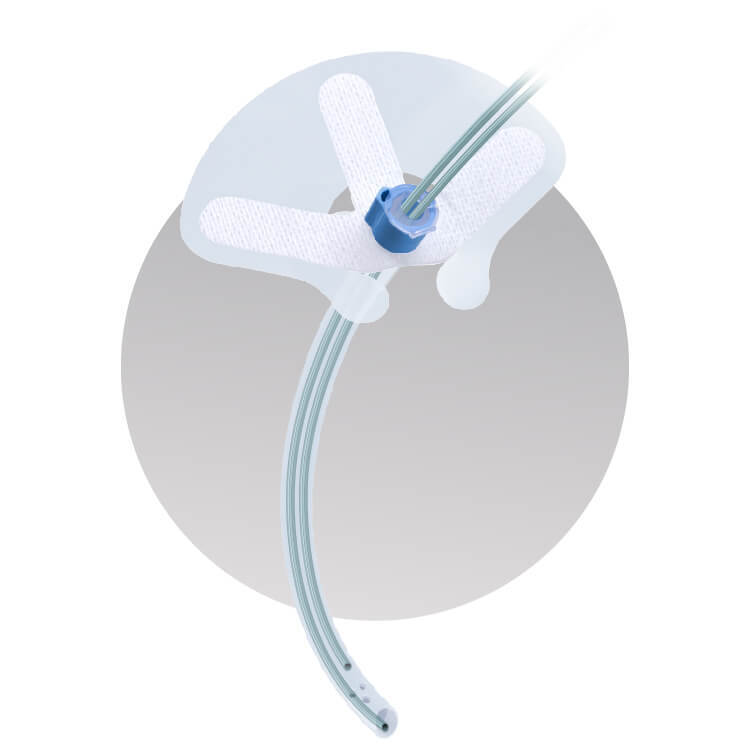
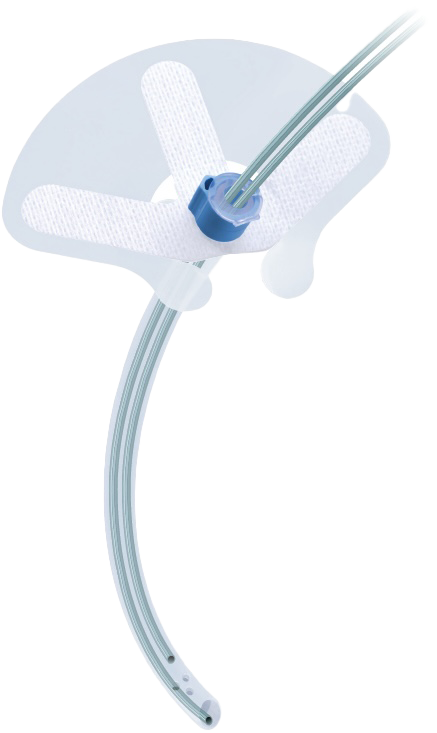
The IntelAir® is a single-use nasal airway support system designed to ensure airway securement, avoid cannula migration, and maintain a clear airway.
- SAFE: Anatomically shaped to reduce insertion trauma, paired with biocompatible silicone promotes patient comfort and oxygen safety.
- SECURE: Designed to avoid tube migration, its anchoring systems keep the airway and cannulas secure – providing consistency in oxygenation and capnography monitoring.
- INTELLIGENT: Integrates airway management, oxygen delivery, and capnography sampling into one streamlined device with a minimal footprint
Click here to download the IntelAir® brochure.
What is a Nasopharyngeal Airway?
A nasopharyngeal airway (NPA) is a flexible tube inserted into the nasal cavity that acts as a temporary airway.
Excellence in Anesthesia Care
The IntelAir® is used during monitored anesthesia to support the soft palate and tongue in sedated patients, keeping the airway patent. Through a simple connection to the anesthesia machine, the IntelAir® allows simultaneous delivery of oxygen to the posterior pharynx and respiratory sampling for end tidal CO2 monitoring. The O2 and sampling tubes are independently adjustable, preventing oxygen washout and providing a reliable end tidal signal.
The IntelAir® features an anatomical shape, designed to reduce bleeding and trauma during insertion. Its thin, medical-grade silicone bolster and gentle adhesive attach to the nose while protecting the ala against pressure injury. Once secured to the face, the IntelAir® provides protection against tube migration, reliable oxygen delivery, and consistent end tidal monitoring.
Part Numbers & Ordering Information
Healthcare Professionals can order with established NET 30 terms or Prepayment/Credit Card.
Frequently Asked Questions
AMT has provided this information as an educational resource tool. This is not intended as a substitute for professional medical care.
Your FIRST source of information should be your healthcare provider.
Is the IntelAir® sterile?
No, the IntelAir® is not sterile.
Can the IntelAir® be sterilized?
No, the IntelAir® is single-use only and is not indicated for reprocessing/sterilization.
What is the MR status of the IntelAir®?
The IntelAir® is considered MR Safe when placed in accordance with the instructions for use.
Do all part numbers include a gas line securement clip?
Yes, all part numbers include a gas line securement clip.
Do all part numbers include a divided O2/CO2 cannula?
No, not all part numbers include a divided O2/CO2 cannula.
What sizes is the IntelAir® offered?
The IntelAir® is offered in a single French size (26F), in lengths of 11 cm, 12 cm, 13 cm, and 14 cm.
What lengths correspond to what French size for traditional NPAs?
There is no standard conversion between length and French size for other NPAs. Across a given French size, NPA lengths can vary as much as +/- 5 cm between manufacturers.
Why is the IntelAir® only offered in one French size?
When sizing an NPA, the length is the most important attribute to ensure proper soft palate support, without extending too far into the larynx. Because larger patients tend to have larger airways, historically NPA diameter would increase with length, to ensure adequate friction to keep the NPA in place. However, larger tubes also require more force, which increases the chance of epistaxis. Since the IntelAir® does not rely upon friction to stay in place, it can utilize a smaller tube – which is intended to reduce insertion-related trauma and be better tolerated by patients in recovery.
What’s included in the packaging?
The IntelAir® is sold in boxes of five, each individually wrapped.
Inside the device packaging is the device (consisting of the nasopharyngeal airway, securement clip, and pre-applied securement tape), a clip opening tool, and a packet of water-based surgical lubricant.
For part numbers that include a divided O2/CO2 cannula, a 22 mm x 6 mm oxygen bushing adapter, and a 7′ divided O2/CO2 cannula are also included in the device packaging.
What are the specifications for the included O2/CO2 cannula?
The length of the O2 supply line is 7 ft. The length of the CO2 sampling line is 7 ft. The O2 flow rate is 6 LPM (rated). The CO2 sampling rate is 0.5 LPM (rated). The CO2 connector is a female Luer-Lock connector. The O2 connector is a push-on trumpet-style connector.
Am I able to purchase the IntelAir® with an included O2/CO2 cannula with a different connector (e.g. male luer-lock, Microstream, etc.)
Different connectors are not available through AMT at this time. However, female luer-lock to male luer-lock adapters and female luer-lock to Microstream adapters are readily available from many suppliers.
Can I use my own O2/CO2 cannula?
Yes, note that the headset tubing of your cannula should have an outer diameter of 0.125 inches (3.2 mm) to ensure compatibility with IntelAir®.
What type of tape is included with the IntelAir®?
The IntelAir® includes a pre-cut and pre-applied tape. The backing is a non-woven polyester fabric with a biocompatible adhesive that is considered gentle and hypoallergenic.
Is the IntelAir® compatible with an Ambu bag or ventilator?
Yes, using a 22 mm x 6 mm oxygen adapter bushing, an Ambu bag or ventilator can be attached directly to the IntelAir™. For IntelAir® part numbers that include a divided O2/CO2 cannula, a 22 mm x 6 mm oxygen adapter bushing is also included with each device.
What are the indications for use for IntelAir®?
The IntelAir® is indicated for use in patients requiring supplemental oxygenation, capnographic monitoring, and/or non-definitive airway support.
What is the approved duration of use for the IntelAir®?
The device is approved for a duration of use less than or equal to twenty-four hours.
 Order Info
Order Info